Clinical Trials Office
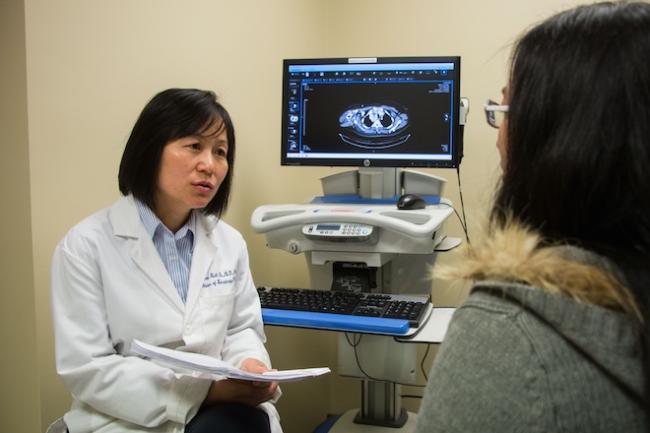
Are you a PATIENT interested in learning more about our cancer clinical trials?
Are you a CLINICAL RESEARCH TEAM MEMBER looking for resources?

Are you a RESEARCH SPONSOR looking for information about our site?
About the Clinical Trials Office
The Georgetown Lombardi Clinical Trials Office (CTO) provides centralized clinical research support to more than 100 Georgetown Lombardi investigators. The CTO provides central management and oversight for coordinating, facilitating, and reporting on cancer clinical trials for Georgetown Lombardi, and education and training of research staff and clinical investigators. It functions as the interface with the Protocol Review and Management System (PRMS), the Data and Safety Monitoring Committee (DSMC), Institutional Review Board (IRB), the Budget and Contracting Offices at Georgetown University Medical Center (GUMC) and JTCC, Office of Research Quality Assurance (ORQA), Disease Groups (DGs), and protocol sponsors to ensure high-quality, safe, and compliant clinical trial conduct.
Cancer clinical trials at Georgetown Lombardi are supported by three clinical locations: MedStar Georgetown University Hospital and MedStar Washington Hospital Center in Washington, DC, and the John Theurer Cancer Center in Hackensack, New Jersey.
Georgetown Lombardi CTO services support the entire life cycle of cancer clinical trials, including:
- Comprehensive study activation and regulatory support from protocol conception to closeout
- Investigational new drug support
- Investigator-initiated trial (IIT) development and conduct
- New trial registration
- Patient screening, registration, and trial participation support
- Financial management
- Data management
Clinical Research Leadership
CTO Leadership

Ernest Richards, PhD, FACN
Administrative Director, CTO NJ





